Why Dwarfism?
In the immortal words of Lennon and McCartney, “I read the news today, oh boy.” By now, you have probably read the news, too, about the discovery of specimens of an extinct, tiny human relative that lived approximately 700,000 years ago on the island of Flores in Indonesia. If not, you can read about it in this SAPIENS article. (It’s okay, I’ll be here when you get back.)
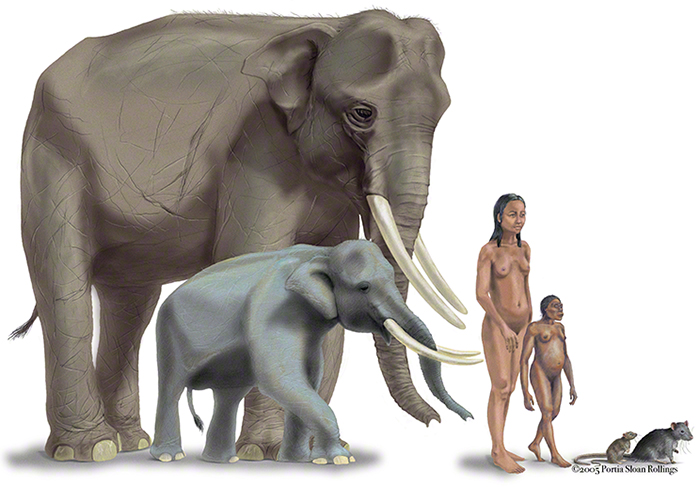
This latest paleoanthropological discovery is exciting for a number of reasons, not the least of which is that it undeniably demonstrates that reduced stature is one way hominins, like other relatively large-bodied mammals, adapt to island conditions. What makes this an indisputable fact is that the remains of other small-statured adult hominins, assigned to the species Homo floresiensis, were previously discovered on the island back in 2003 at a fossil cave site called Liang Bua. A recent analysis of the deposits at Liang Bua cave dated those fossils to between about 100,000 and 60,000 years ago. Some researchers suggested the Homo floresiensis specimens were a pathological form of modern human, rather than a new hominin species, but the latest finds, from a site called Mata Menge, put that debate to rest—modern humans evolved in Africa only 200,000 years ago and, as I mentioned, the tiny Mata Menge hominins inhabited the island at least 700,000 years ago. And the odds of excavators uncovering specimens solely of diseased individuals, with morphological similarities and from such temporally divergent sites, are astronomically small.
Both the Liang Bua and Mata Menge hominin fossils are evidence of “insular island dwarfism” among hominins. I should note that “dwarfism” in this context does not refer to the congenital disorder, achondroplasia, of a single individual but to an evolutionary phenomenon in which, over generations, body size is reduced in a population of organisms. For island dwellers over a certain size, body size can reduce significantly over time—and often quite quickly, in as few as 2,000 to 3,000 years.
For example, take the case of the endangered dwarf foxes of the California Channel Islands. The living island foxes’ larger-bodied grey fox ancestors are thought to have colonized the northern Channel Islands as much as 9,000 years ago as a result of rafting or human introduction. The foxes, which weigh just 1 to 3 kilograms (roughly 2 to 7 pounds), currently occupy six of the islands where they are one of only 10 species of land mammal. Courtney Hofman and colleagues suggest that island dwarfism of the foxes may have occurred in as few as 2,000 years, as fox bones found at the earliest archaeological site—dated to about 7,000 years ago—were already smaller than their mainland grey fox relatives.
Recently, a researcher discovered a new population of dwarf lemur living on a small island in far northern Madagascar. These rare primates are likely descendants of other dwarfed lemurs living about 65 kilometers (about 40 miles) away in the western dry forests of the main island of Madagascar. Like the Channel Islands foxes, the lemurs could have drifted from one island to the other by rafting. Though it’s not known precisely when these petite primates first landed in this pristine place, free of human inhabitants, they exhibit particular signs of a population that has been isolated on an island for more than a few generations. Not only are they small, they lack a fear of predators—signs they have grown used to a predator-free lifestyle.
A scarcity of predators may have a lot to do with why some island populations get smaller over time. If an animal does not need to be large to flee or defend itself from predators, then predation pressure is relaxed and body size can safely decrease over time. Other factors that may play a role are resource abundance, competition levels, and energetics.
The two primary models that explain how larger-bodied species may become dwarfed in an insular environment involve a relaxation of predation and competition pressures—but each emphasizes a different driving force behind the actual reduction in body size.
The first model assumes that because island environments are structured differently than mainland environments, the ability for island species to exploit resources as effectively as they did on the mainland is diminished. This may be due to a lack of variety or an absence of high-quality resources on the island, or simply too few resources for a population of large-bodied animals with limited ability to migrate (due to island geography). With limited or low-quality resources, large animals might experience stunted growth and lower reproductive rates. If those populations begin to produce smaller individuals that require fewer or lower-quality resources, they may have a better shot at maintaining or improving their reproductive fitness. Thus, the model says that island dwarfism results from a need to counteract the detrimental effects of poor or restricted diets, particularly for dietary specialists. If the population also experiences competition, this process may happen more quickly.
The alternative model, though not mutually exclusive to the first, predicts that if a population’s usual competitors and predators are absent, there will be natural selection for an optimal body size for energy acquisition and use, and this selection pressure will be the driving force behind the phenomenon of dwarfism. Optimal body size is the point at which there is an ideal energetic trade-off between getting and using resources (for example, for growth) and producing offspring.
In the Spinagallo caves of Sicily, more than 100 specimens of the smallest elephant that ever lived, Elephas falconeri, were recovered. Scientists studied the remains to assess whether it was more likely the species dwarfed due to overcrowding and an ecologically restricted environment or due to selection for optimal body size. They made the observation that these ancient elephants, 150 times smaller than large Elephas species, developed faster (reached sexual maturity earlier) and had relatively higher offspring mortality rates than larger elephants. They noted that organisms with fast growth rates tend toward high juvenile mortality rates, which increases selective pressure for an increase in the frequency of births. And since the scientists documented a large percentage of juveniles among the fossils, they assumed E. falconeri’s reproductive output was high. It takes energy to reproduce, so if rates of reproduction increase, energy expenditures do, as well. The scientists hypothesized that the increase in energy required for earlier, faster reproduction was offset by a decrease in energy needed for growth—because the elephants were getting smaller.
The researchers also noted that some E. falconeri females lacked tusks and that the adults had morphological features indicative of paedomorphosis (a retention of juvenile-like traits). Both of these findings suggest that energy typically used for growth and development was being reallocated for reproduction. The researchers ultimately settled on the idea that the elephants were evolving toward an optimal body size and that their dwarfism was not simply a result of poor or restricted resource availability.
On the island of Flores, another proboscidean, Stegodon, became dwarfed—possibly more than once—and later went extinct. A pygmy Stegodon population may have lived until around 900,000 years ago when it was possibly hunted out of existence by newly arrived hominins (stone tools from Mata Menge are thought to be about 950,000 to 810,000 years old). Evidence of another pygmy Stegodon population found in the much younger Liang Bua cave deposits may have survived until approximately 12,000 years ago when it would have been forced to extinction by a volcanic eruption.
If Stegodon was, like elephants, an effective generalist when it came to diet, then resource deprivation probably was not the driving factor behind its reduction in stature. Notably, most of the Stegodon fossils from Liang Bua are juveniles, a pattern seen in the study of E. falconeri, which would suggest high reproductive output and fast growth rates associated with high juvenile mortality.
Did the hominins of Flores also experience insular island dwarfism as the result of selection for optimal body size? Or was their reduced stature a contingency of limited or poor-quality resources? The factors we have to consider, in addition to resources, are predation, competition, growth, and development. It has been suggested that Homo erectus used watercraft to initially reach the island and that the Flores hominins were their descendants, so it is important to consider what is known about Homo erectus growth and development.
The only potential hominin predators on Flores are the Komodo dragon and another varanid. Today, Komodo dragons on the island of Komodo, near Flores, eat “any and all of the other large animals on the island, including wild boar, deer, water buffalo, dogs and goats. If hungry, a Komodo will eat snakes, birds, and even smaller Komodos.” The bite of a Komodo is extremely poisonous and deadly. These large reptiles have been clocked at speeds up to 20 mph. Clearly, a small Flores hominin would be susceptible to being attacked by a Komodo, but they potentially could have avoided encounters with these large predators or were able to defend themselves with tools or weapons. Examining Komodo bones for evidence of cut marks would be worthwhile to determine whether the hominins defended themselves against, or even hunted, these animals. If we could conclude that the hominins were able to defend themselves against these large predators, or that veranids seldom attacked hominins, we could assume that predation pressures were low on Flores.
Regarding competition, scientists report that the ancient fauna of Flores included monkeys, deer, and birds. If the Flores hominins were greatly dependent on fruits, these animal species may have been competition for them but only during certain times of the year. Hominins in the genus Homo tend to be more dietary generalists than specialists, so it is unlikely that the Flores hominins experienced significant competition with other species for resources. And because the hominins had stone tools, it is possible that they were hunting and eating their potential prime competitors.
Analyses of the growth and development of the Nariokotome skeleton of Homo erectus, based on the dentition, have concluded that this hominin species had a more ape-like (versus Homo sapiens–like) growth trajectory. This means that Homo erectus individuals grew faster and became sexually mature earlier as compared to modern humans. The Nariokotome skeleton shows an estimated stature of at least 6 feet for that individual and an age estimate of only 8 to 12 years. If Homo floresiensis evolved from a Homo erectus ancestor, it is possible that the first population of Homo erectus to inhabit this island had to maintain fast individual growth and development rates. This suggests that there must have been high-quality resources (like fruits and Stegodon) available to Homo erectus; otherwise, that original population would have suffered and trended toward lower reproductive rates, not higher. What follows is that in an environment with relaxed predation pressure, low competition levels, and high-quality resources, the hominin populations that inhabited Flores simply would have no reason to be big and could evolve toward an optimal body size and succeed on an island for 60,000, if not 900,000, years.
So why did the diminutive Flores hominins go extinct? As Courtney Hofman and colleagues point out in their article about the dwarf island foxes, “small populations of island endemic taxa are often at risk of extirpation or extinction due to their reduced genetic diversity and increased susceptibility to genetic drift, disease, and climate change, especially in conjunction with over-exploitation, habitat loss, and predation or competition from invasive species.” Did disease or climate change drive the Flores hominins to extinction (possibly more than once)? If not—and predation pressure and competition were low for so long—what could have changed for the populations that lived at Mata Menge and Liang Bua? Perhaps for the Liang Bua hominins, the competition got heavy when a new wave came on shore—carrying Homo sapiens.
The remaining question is: How many times did hominin populations on Flores experience island dwarfism? In other words, are the Liang Bua hominins descendants of the Mata Menge hominins, or are they the result of a later migration of large-bodied hominins who also evolved a small stature? Only time, and more data, will tell. What do you think?